Pharmaceutical logistics, a rapidly expanding market
The global pharmaceutical logistics market is booming, across the globe, also due to the impact of the pandemic. After reaching $ 69 billion in revenue in 201 according to a new report by Grand View Research, the global pharmaceutical logistics market will have an annual growth rate of 7.3% through 2027.
The Role of Logistics in the Pharmaceutical Industry
Pharmaceutical logistics deals with the storage and distribution of medicines, biological active substances from the supplier to the final point of sale (POS). At the end of the supply chain there is always the customer or patient who needs care. It is therefore easy to understand that storing and handling pharmaceutical goods involves great responsibilities, strong skills and above all high Quality and Safety Standards.
Punctuality, accuracy and traceability: these are the good practices of the pharmaceutical logistics service
The purpose of Pharmaceutical Procurement and Supply Management is that products are available in the right quantity, at the right time, with the right service.
For this reason, pharmaceutical logistics operators are required to strictly observe European (GDP – Good Distribution Practices – Guidelines of 5 November 2013 – Guidelines of 19 March 2015) and Italian (Ministerial Decree 6 July 1999) regulations relating to good distribution, including:
Maximum traceability and inventory control: to give possibility of a quick inventory of irregular or defective drugs batches;
Accurate quality control: accurate quality control: every pharmaceutical logistics company must comply with a rigorous Quality System that involves the entire organizational structure, environments, processes, procedures, human resources management, technologies and activity used;
Controlled temperature and relative humidity monitoring system for storage and transport: temperature controlled cold stores, quarantine areas, delivery vans, containers and packaging are essential to avoid any contamination and maintain the cold chain when dealing with heat-sensitive vaccines and medicines;
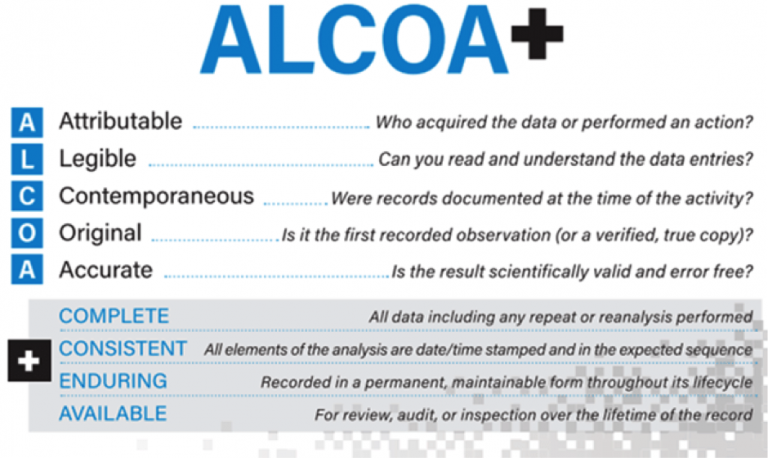
Data tracking: both the pharmaceutical production sector and the pharmaceutical logistics sector must strictly respect the principles of “Data Integrity”. Every procedure done in the inbound, storage, pick & pack and outbound phase have to be simultaneously recorded and supervised. You must have the proper documentation to prove your procedures during an audit: “If you can’t prove it, you haven’t.” This is way it is considered a “must” to approach any aprocedure following the “ALCOA + principles”:
Reduced delivery times: time is very important in this industry, as the valuable pharma products handled, often require urgent delivery;
Transport validation process: specific protocols based on risk analysis and transport simulations have to be designed in order to validate the routes (Route Validation).
The world of logistics is already quite articulated and complex and in pharmaceutical logistics becomes even more complicated.
According to the Supply Chain Manager Ivan Sannino MD of IS Consulting, today the most important challenges for those involved in the acceptance, installation, storage, order preparation and transport pharmaceutical logistics sector is “knowing how to combine new technologies and the cold chain with a complex set of compliances that regulate the distribution of the drug.”